Nitrogen Management: Minimizing Losses and Greenhouse Gas Emissions with SWAT MAPS

If 10% of the fuel you put in your tractor disappeared, would you want to know why? What if sometimes it was all accounted for, and sometimes you lost 20%? Would you want to understand why? Of course you would!
As an agronomist working with SWAT MAPS, I feel the same way about nitrogen. We have seen many examples over the past few years of soil nitrogen levels varying dramatically by SWAT zone due to either very wet or very dry conditions. The urge to understand why and be able to explain it is insatiable.
The challenge is that the nitrogen cycle is far from simple, and as a result it can be tempting to ignore a lot of the details and keep doing what we are doing. But there are some nitrogen behavior principles that can help us make dramatically better nitrogen decisions – we just have to understand them. Here are a few important facts about nitrogen that everyone should understand:
1) Nitrogen in the soil is constantly cycling. Fertilizer N is not simply converted to nitrate and then either taken up by the crop or lost. A large portion of fertilizer N is used by microbes for their energy needs too. This is called immobilization, or sometimes referred to as “tie-up.” It doesn’t mean it is lost; it is just stored in the soil in an organic form that will become available later (months or even years) through mineralization. A certain portion of nitrogen that a crop takes up is really nitrogen that was applied to previous crops, was temporarily immobilized, and now mineralized again as the organic matter decomposes.
So, just because you read that a crop only uses 50% of the nitrogen you apply in the year it was applied, doesn’t mean the rest is lost! In most dryland environments, much of it stays in the soil in some form to be used later – although whether it is still there at a later date depends on a few things...
2) There are several mechanisms of loss and they can all happen in the same field in the same year. This sounds ominous, but the more we understand these mechanisms, the less daunting it feels to manage these losses.
Leaching of nitrates occurs under very wet conditions in freely draining soil — sandier soil types that don’t hold a lot of water — and as a result water moves down through the profile, below the crop rooting zone, and takes dissolved nitrate-N with it. In some areas, this can cause nitrate accumulation in aquifers and can be a serious problem for drinking water quality.
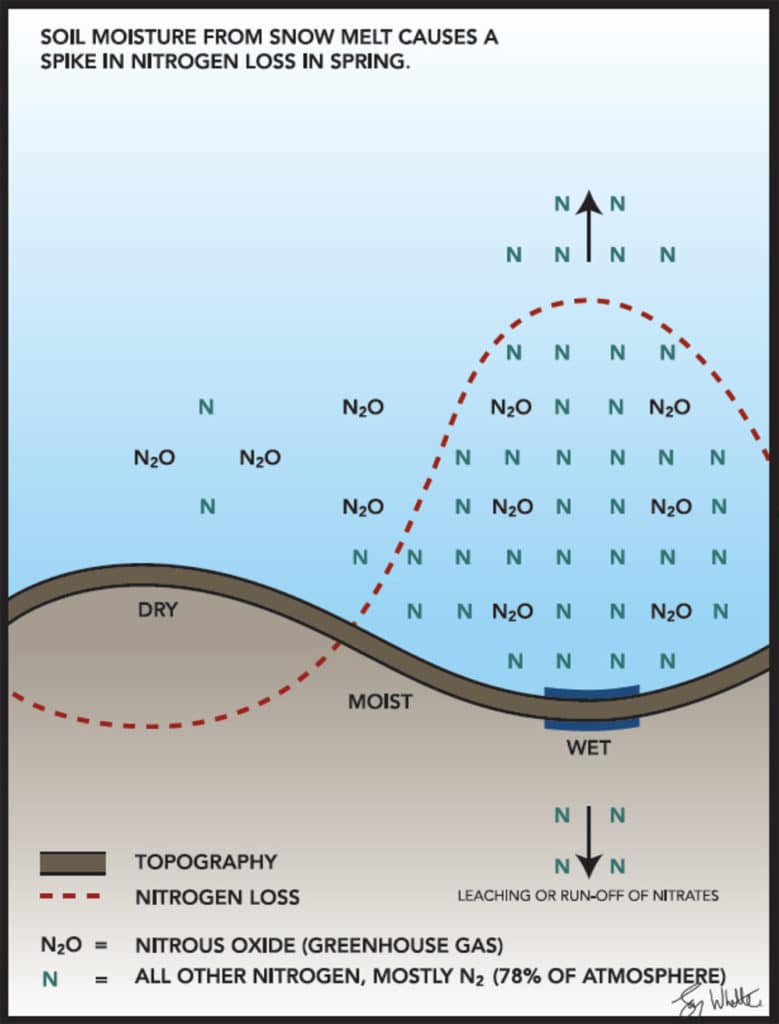
Denitrification is another mechanism of N loss that occurs in wet soils, but more so in poorly drained soils that are relatively saturated (little to no air-filled pore space). Fine textured clays and sodic soils are often most susceptible to these losses, especially in lower landscape positions that collect runoff water after large rains or snowmelt (refer to Figure 1). This loss mechanism sees nitrate-N converted to nitrous oxide (N2O) and dinitrogen gas (N2). While both forms are lost and can no longer be used by the crop, N2O is also a potent greenhouse gas, so governments and climate scientists around the world are particularly interested in how we can reduce N2O emissions from fertilizers in agriculture.
Volatilization is the third primary mechanism of nitrogen loss, which occurs mainly when nitrogen fertilizers or manures are surface applied or shallow banded, and ammonia-N is able to ‘gas-off’ into the air rather than be held by soil particles. Volatilization can be prevented by deep banding, using nitrate-N fertilizer sources, or using urease inhibitors that slow the conversion of urea to ammonia. Read more about the use of inhibitors here.
3) Loss mechanisms are driven by soil, water, and topography. Where this gets interesting is how we can leverage our knowledge of N loss mechanisms by understanding the factors that drive them, which are mainly soil texture, water, and topography. Figure 1 depicts typical loss patterns across a hummocky landscape with topography. During snow melt or after large rain events, water sheds off hills, flows into depressions, and causes saturated soil conditions. If the depression is well drained with sandier soil, this will cause leaching. If it is poorly drained and causes water ponding, losses via denitrification will likely be greater than leaching, adding up to several kg of lost nitrogen per day in warm soils! In completely saturated conditions much of the nitrate can be lost as N2. In the toe-slope positions this may shift to a larger amount of N2O, while further upslope losses diminish to much smaller, insignificant amounts. The point is, losses are not equal across the landscape, and if we better understand soil, water, and topography variability, we can better understand what losses are occurring, where, and why.
What can we do? Thankfully the basics of 4R nutrient stewardship still apply, but to take it to the next level of management we need to consider those 4Rs spatially, not just on a ‘field average’. You must map the soil, water, and topography variability, then soil test different areas: sands vs clays; hills vs mid-slopes vs depressions; and well-drained depressions vs poorly drained depressions vs saline depressions. This is why SWAT MAPS are such a valuable tool for nitrogen management, because it considers all these factors in a single map. Yes, it takes some effort and expense, but the rewards go far beyond nitrogen management.
One of the most common culprits of poor nitrogen utilization and emissions is excess nitrates accumulating in saline soils, sodic soils, and high organic matter (>10%) depressions. Millions of acres of SWAT soil samples have shown us that these areas often have excessive soil nitrate levels that are highly susceptible to losses. Simply using variable rate to reduce applied rates on these soils can not only save money but reduce N2O emissions. Further management with enhanced efficiency nitrogen sources or alternative application timing can also help improve the economic and environmental outcome of farming these challenging soils. It really comes down to getting more nitrogen into the crop instead of the air or water around us.
It is important to understand that while a lot of focus remains on N2O emissions, they are only a small fraction of total N losses in normal agriculture systems, typically less than 2 kg/ha/yr. The reason N2O gets attention is its potency as a greenhouse gas, and because man-made emissions from fertilizer have been increasing. Since N2O is a product of both nitrification and denitrification, we can help reduce N2O emissions by:
- Not using more nitrogen than necessary, reducing nitrification derived N2O. Soil tests and fertilizer or seeding rates based on realistic SWAT zone yield targets are valuable tools.
- Reducing denitrification risk by managing N rates by SWAT zone (specifically focusing on SWAT zones 6-10 that are relatively wet), using enhanced efficiency nitrogen products, splitting N applications, or combinations of all.
We cannot expect to eliminate nitrogen losses or emissions. This is not an “all or nothing” approach. But we are making steady progress in understanding losses and ways to manage them, and that knowledge will guide us to a more sustainable future we can all be excited about and proud of.
Wes Anderson
VP of Agronomy
wes@swatmaps.com
References
Dunmola, A. S., Tenuta, M., Moulin, A. P., Yapa, P., & Lobb, D. A. (2010). Pattern of greenhouse gas emission from a Prairie Pothole agricultural landscape in Manitoba, Canada. Canadian Journal of Soil Science, 90(2), 243-256.
Fiedler, D. J., Clay, D. E., Joshi, D. R., Engel, A., Marzano, S. Y., Jakubowski, D., ... & Clay, S. A. (2021). CO2 and N2O emissions and microbial community structure from fields that include salt‐affected soils (Vol. 50, No. 3, pp. 567-579).
Ghosh, U., Thapa, R., Desutter, T., Yangbo, H. E., & Chatterjee, A. (2017). Saline–sodic soils: potential sources of nitrous oxide and carbon dioxide emissions?. Pedosphere, 27(1), 65-75.
Glenn, A. J., Moulin, A. P., Roy, A. K., & Wilson, H. F. (2021). Soil nitrous oxide emissions from no-till canola production under variable rate nitrogen fertilizer management. Geoderma, 385, 114857.
Smith, C. J., Hunt, J. R., Wang, E., Macdonald, B. C., Xing, H., Denmead, O. T., ... & Zhao, Z. (2019). Using fertiliser to maintain soil inorganic nitrogen can increase dryland wheat yield with little environmental cost. Agriculture, Ecosystems & Environment, 286, 106644.









